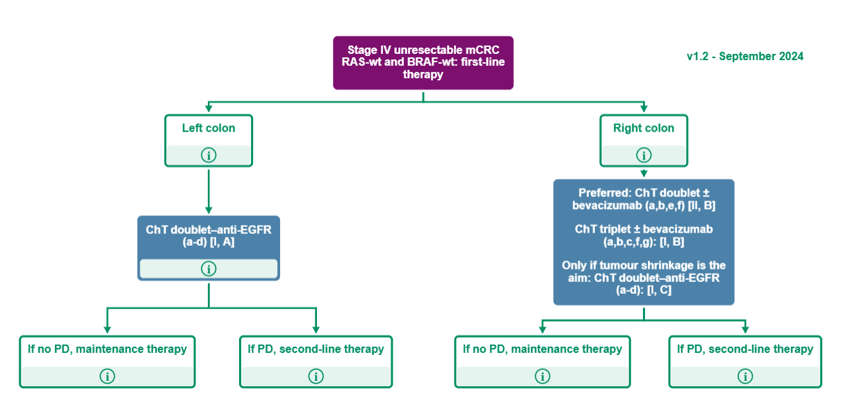
Purple: general categories or stratification; blue: systemic anticancer therapy; white: other aspects of management.
5-FU, 5-fluorouracil; CAPOX, capecitabine–oxaliplatin; ChT, chemotherapy; EGFR, epidermal growth factor receptor; FOLFIRI, leucovorin–5-fluorouracil–irinotecan; FOLFOX, leucovorin–5-fluorouracil–oxaliplatin; FOLFOXIRI, leucovorin–5-fluorouracil–oxaliplatin–irinotecan; MCBS, ESMO-Magnitude of Clinical Benefit Scale; mCRC, metastatic colorectal cancer; PD, progressive disease; PS, performance status; S-1, tegafur–gimeracil–oteracil; wt, wild-type.
(a) In patients presenting with cardiotoxicity and/or hand-foot syndrome on 5-FU or capecitabine-based ChT, S-1 may be used as an alternative [III, B].
(b) Additional details on treatments and drug combinations can be found under the section ‘Management of advanced and metastatic disease without potential conversion’ (subsections ‘First-line treatment’ and ‘Second-line treatment’).
(c) FOLFIRI–cetuximab ESMO-MCBS v1.1 score: 4; FOLFOX4–panitumumab ESMO-MCBS v1.1 score: 4.
(d) FOLFOX4–panitumumab ESMO-MCBS v1.1 score: 4; for FOLFIRI–cetuximab ESMO-MCBS v1.1 score: 4.
(e) In a very selected population.
(f) CAPOX– or FOLFOX4–bevacizumab ESMO-MCBS v1.1 score: 1.
(g) A triplet with FOLFOXIRI plus bevacizumab is an option for selected patients with good PS and without comorbidities.